Photonic technologies are set to revolutionise our access to chemical and biochemical information, driven by the demand for fast, low-cost, automated chemical analysis in a multiplicity of applications from food safety, water quality, security, personal and preventative medicine, pharmacogenetics and rapid point-of-care diagnostics.
The scale of integration, low cost and robustness of microfabrication approaches which have enabled the ubiquitous presence of the mobile phone and digital camera are expected to lead to similarly widespread deployment of chemical and bioanalytical microsystems. Optical techniques have traditionally played a major role in quantitative chemical analysis and remain the mainstay of detection in “lab-on-chip” systems, but the degree of optical functionality integrated within these systems remains extremely limited, and they have yet to benefit fully from the massive growth in photonics telecommunications technologies in recent decades. Photonic technologies for telecommunications operate in the near infra-red (NIR) wavelength region from 0.8μm – 1.8μm, driven by the spectral transmission window in silica optical fibre. The ideal molecular “fingerprint” region for biochemical analysis is dominated by the mid infra-red (MIR) spectral region from 2μm - 13μm. Biosensor and lab-on-chip research and commercialisation have both been severely hampered by the lack of an integrated photonic platform which can operate over both the NIR and MIR spectral ranges, and which would enable new opportunities for sensitive, selective, label-free biochemical analysis. This programme sets out to advance the frontiers of biophotonics research in MIR materials systems, integrated photonic components for biochemical analysis and nanostructured photonic materials for light control. New approaches to clinical point-of-care diagnostics will be enabled by realising a mass-manufacturable monolithically-integrated photonics/optofluidic technology for chemical and biochemical analysis in the near and mid-infrared, exploiting advanced spectroscopic techniques for accessible biomedical diagnostics.

The WIPFAB project is funded by the European Research Council under the EU 7th Framework Programme (FP/2007-2013) / ERC Grant Agreement no. 291216.
The project themes include:
1 NIR/Mid-IR waveguide materials
2 Photonic tools on a chip
3 Nanostructures for surface intensity enhancement
4 Diagnostic applications
1 NIR/MIR waveguide materials
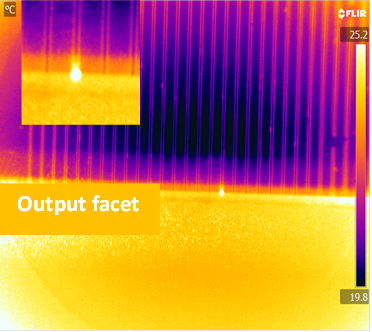
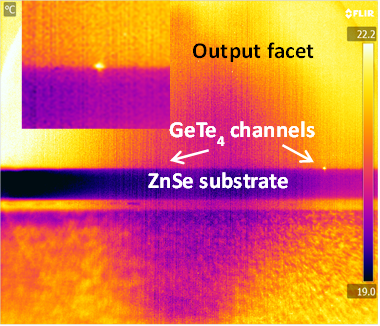
To realise a wideband integrated photonic circuit technology for molecular fingerprint absorption spectroscopy for wavelengths up to and beyond 15 µm, a range of mid-IR materials was evaluated and germanium telluride from the chalcogenide glass family was selected for wide transmission window, high refractive index and chemical/mechanical robustness. Ta2O5 was selected for transmission up to 8-10 µm, ease of processing and CMOS compatibility. GeTe4 glass waveguides have been deposited as thin films on zinc selenide substrates and on silicon wafers with a selenide low index buffer layer. A detailed materials analysis of these deposited films has been carried out and photolithographic patterning of these films has resulted in channel waveguides which have successfully guided light in the mid-IR from 2.5 - 3.7 µm. Work is ongoing to characterise these in the 4-14 µm wavelength range. Waveguide designs have been theoretically optimised for maximum surface sensitivity to adsorbed biomolecular layers. Ta2O5 waveguides have been successfully realised on CaF2 substrates (needed for mid-IR transmission) using a new low-temperature process and initial trials of using these for studies of biological samples are underway. A specialised mid-IR characterisation facility, based around quantum cascade lasers (QCL) and an optical parametric oscillator (OPO), has been established to provide tunable sources of coherent light over an uninterrupted 2.5-14 µm range. A specialised FTIR spectrometer has been adapted for waveguide measurements at wavelengths beyond this range. Improvements are still required in the processing and characterisation of these mid-IR waveguides, but they offer a very promising low-cost mass-producible platform for ultrasensitive spectroscopy of biochemical analytes, and potentially have broad application in other optoelectronic applications where the mid-IR is of increasing interest.
| OPO based waveguide characterisation apparatus (2.5-3.7μm) |
| Top view of the GeTe4 waveguide showing guided light at the output facet (3.7μm) |
| QCL based waveguide characterisation apparatus (6-12μm) |
| Cross section of the GeTe4 waveguide showing guided light at the output facet (3.7μm) |
2 Photonic tools on a chip
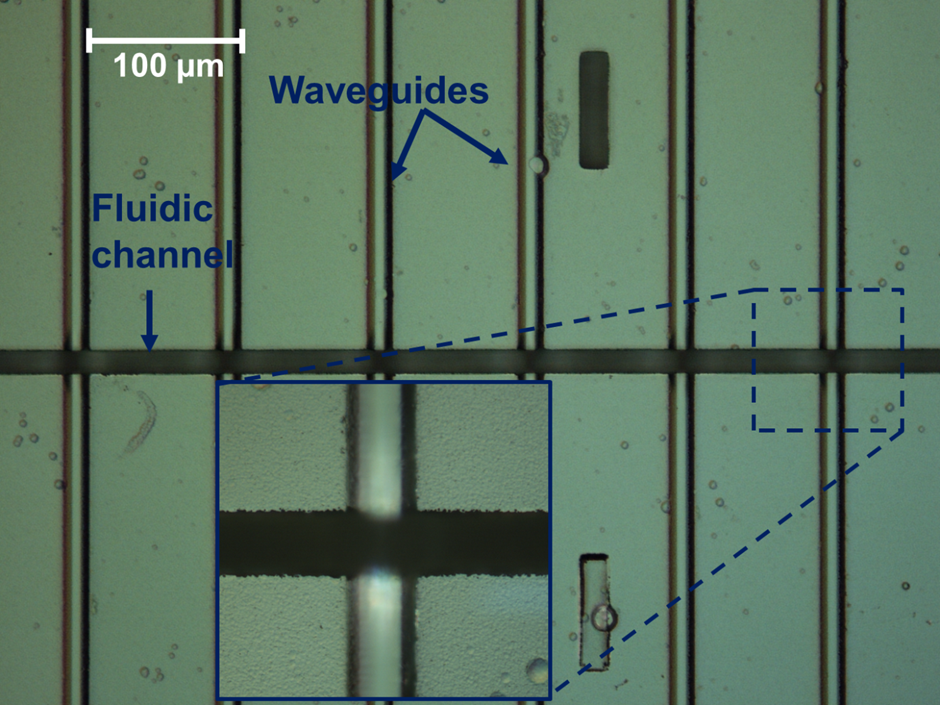
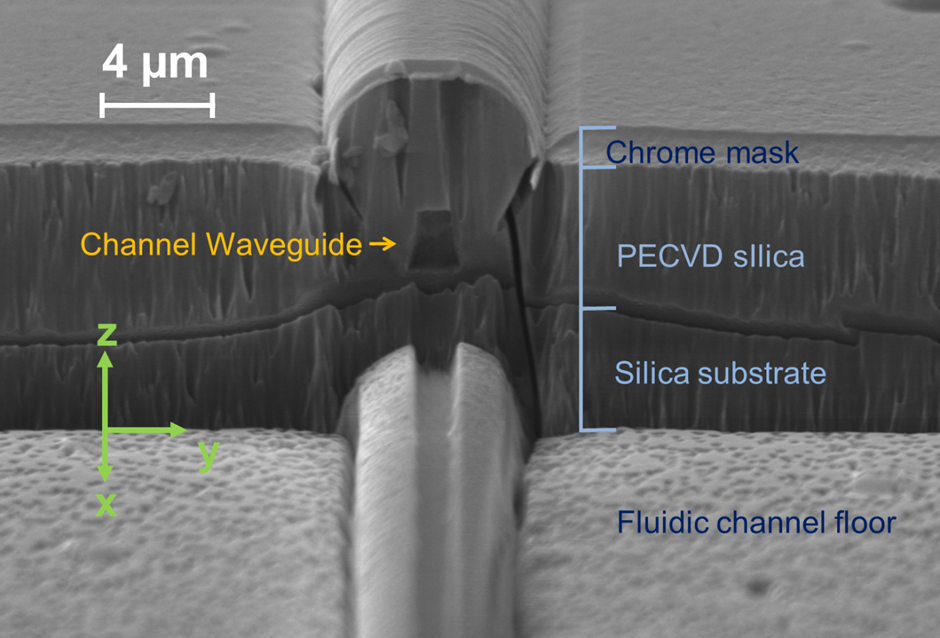
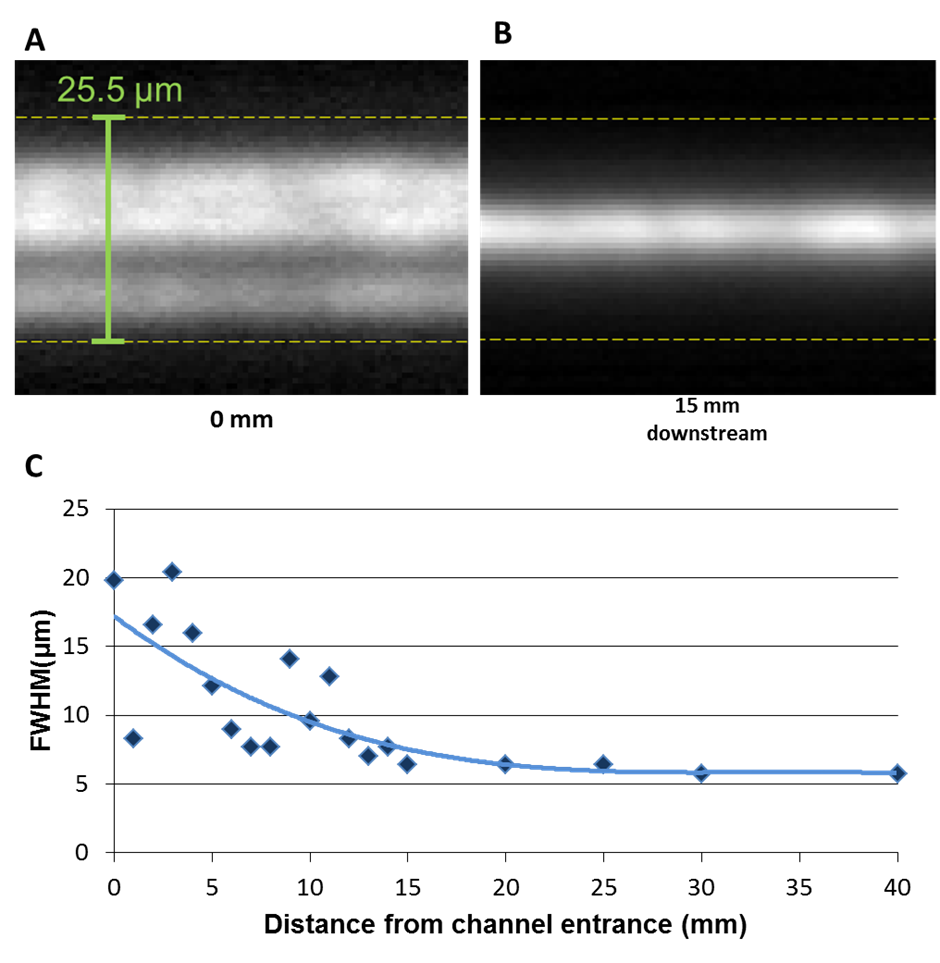
In response to engagement with Southampton General Hospital (SGH) and Southampton University Centre for Biological Sciences, work on this topic has centred around realising a monolithically integrated flow cytometer chip for multiplex immunosensing on beads. An integrated optical platform offers a compact alternative to free space optics used in traditional flow cytometers, cutting costs and reducing complexity. Visible wavelengths are used due to the nature of the commercial fluorescent beads and the fluorescent biochemical tags. An integrated waveguide/fluidic platform technology has been established, incorporating arrays of Ge-doped silica waveguides buried deep (~15 µm) below the surface of the chip and crossed perpendicularly by a ~30 µm deep by ~15 µm wide fluidic channel. The channel geometry causes inertial focussing of the beads into streams passing at mid-height in the channel, allowing repeatable and efficient excitation by light emerging from the waveguides though the channel wall and collection of scattered light from waveguides in the opposite wall, without the need for complex sheath flow or electrokinetic focussing. Technological advances have included realisation of low-loss monomode waveguides for green and red wavelengths, deposition of a low-loss thick cladding, and deep reactive ion etching of >30 micron deep fluidic channels through the cladding, waveguide and into the substrate, with excellent perpendicularity (93°) and surface quality, resulting in a successful measurement of cross-channel transmission with a narrow beam diverging to a maximum width of 4.2um at the far channel wall and the fluorescence signals from hundreds of calibration beads. The device will initially be used to perform immonassays for the cytokine TNFα, a messenger molecule involved in the inflammatory response of the immune system and of broad interest to researchers in the areas of neurodegeneration and stroke recovery. This integrated optofluidic platform will enable a wide range of uses in bioanalysis, the first of which will be for rapid multiplexed immunoassays in the doctor’s office without the need for large, complex instruments needing significant skilled technical support.
| Top down microscope view of the fluidic channel crossing the array of waveguides |
| SEM image of the side wall of the fluidic channel showing the etched end facet of an integrated channel waveguide |
| Transmission fluorescence microscope image showing the summed image of multiple fluorescent streaks left by calibration beads passing down the microfluidic channel. The beads are focussed into a tight stream at 15mm from the channel entrance |
3 Nanostructures for surface intensity enhancement
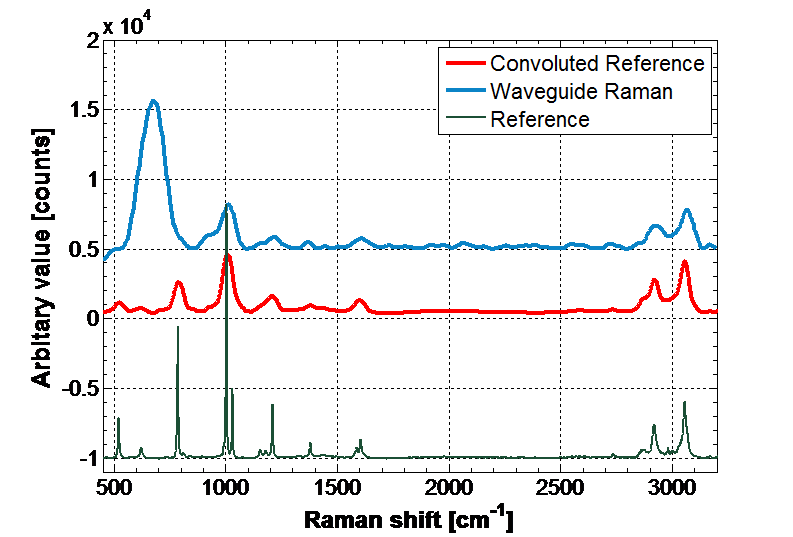
Achieving low limits of detection through spectroscopic analysis of small quantities of biomedical analytes in monomolecular surface films at low cost can be challenging due to weak absorption and emission cross-sections and low numbers of molecules. Nanostructuring materials at the scale of the wavelength of light and below can transform their optical performance. The most striking example of this in chemical analysis is the nanostructuring of a gold surface for surface-enhanced Raman spectroscopy (SERS), which can achieve “amplification” of Raman signals from molecules at surfaces by factors of up to 108 through the excitation and localisation of surface plasmons (SPs). Less well known is the technique of surface-enhanced infra-red absorption spectroscopy (SEIRAS), which relies upon a similar localisation phenomenon. These techniques are ideally suited for integration with optical waveguides and we have already demonstrated high-efficiency waveguide excitation of SPs. We aim to take this forward to “SERS chips” for applications such as to respiratory infection and detection of single nucleotide polymorphisms (SNPs) and short tandem repeats (STRs) in DNA analysis.
Composite plasmonic waveguide simulation
Theoretical models have been established to underpin experimental research into nanostructured waveguides for enhanced spectroscopies, including a transfer matrix model comparing conventional and waveguide excitation of Raman at dielectric and gold-coated surfaces, a finite-element based approach to study the surface intensity in composite metallo-dielectric waveguides and an FDTD model to study waveguide nanostructure excitation.
SERS chips for biochemical sensing
All-dielectric enhanced Raman chips are being explored to realise high-sensitivity detection in a mass-producible format with low-cost instrumentation. These are complementary to the metallo-dielectric plasmonic devices and represent a compromise between the lowest detection limits and the greatest reproducibility.
Waveguide surface-enhanced infrared absorption spectroscopy
Enhancement mechanisms in surface-enhanced infrared absorption spectroscopy (SEIRAS) are less well advanced than for SERS, and simulations are being used to explore optimising devices for SEIRAS. Waveguide absorption spectroscopy experiments in the NIR to establish baseline sensitivity have shown strong enhancement of the spectrum. Mid-IR SEIRAS waveguides are expected to yield ultra-sensitive detection of low concentrations of biomolecules.
4 Diagnostic applications
WIPFAB diagnostic applications are driven by the clinical needs of the School of Medicine, University Hospital Southampton and the wider medical community. Our combination of optics, materials science, microfluidics and spectroscopy expertise is hugely empowering. It allows us to adopt different sensing methodologies in order to extract useful information from varied sample media as quickly and efficiently as possible. Some examples include:
Multiplexed near-infrared immunoassays in novel biological media
In an attempt to move away from conventional blood samples and their associated problems with fluid handling and coagulation, the diagnostic value of basal tear fluid is being investigated to see if it can be a viable and more easily obtained alternative. Of particular interest is the diagnosis of ocular disease and long-term monitoring of treatment efficacy.
Characterisation of drugs in complex aqueous media
The chemical structure of many drugs make them particularly convenient for mid-infrared (MIR) characterisation using the spectroscopic fingerprint region. Real-time pharmacological data on drug metabolism for inferring patient uptake can be obtained by combining MIR optics with microfluidic sample handing, which could allow clinicians to make more informed choices during surgery compared with conventional monitoring equipment.
Stem cell differentiation
We have successfully used FTIR spectroscopy toassess the differentiation of stem cells according to the conditions in which they were grown in vitro. The critical advance is in monitoring wet samples, not dried. We aim to translate FTIR spectroscopy from end-stage assessment to real-time bench-top monitoring by exploiting the potential of MIR optics for miniaturisation and mass production.